🎄12 Days of HPC
On the 4th day of christmas the HPC gave to me: An expansive computational sweep of the relevant experimental design parameters in order to increase our understanding of the formation mechanisms of globular protein-based hydrogels...in a pear tree
During the month of December we’re featuring blog posts from researchers from across the University of Leeds showcasing the fantastic work they do using our High Performance Computing system. Follow us @RC_at_Leeds to keep up to date with our 12 days of HPC blog series.
What’s your name?
Benjamin S. Hanson
What department do you work in?
Physics and Astronomy
What research question are you trying to answer?
The dynamical formation processes and subsequent mechanical responses of protein-based hydrogels are of significant scientific interest, as the ability to rationally design these objects will lead to an era of “smart” biomaterials with a wide range of applications. We are trying to use mesoscale simulation techniques in parallel with experimentation to determine how these protein networks grow, and how this yields their subsequent mechanical response.
How does HPC help your research?
The ability to access a large amount of parallel computing power and almost immediate and efficient allocation and return of physics calculations that would be unfeasible on either a single work station, or multiple work stations with different confiurations.
What is the potential impact of your research?
Insight into the hierarchical mechanics and statistical mechanical processes at the biological mesoscale will lead to a more thorough understanding of biological physics in a very recently accessible experimental regime. In addition, the specific systems we study have a wide-range of applications in medicine, from drug-delivery to accelerated wound healing and artificial biological scaffolds.
In your personal opinion what’s the coolest thing about your research?
The philosophical insights into what it truly means to do science are completely apparent at the biological mesoscale. Here, empricial experimental induction and theoretical deduction are on the verge of meeting, and the ideal cross-over point for this is computation, which combines numerical deduction with experimental variation. And it all happens in biological systems, so we are using life itself as a focus to do relatively abstract science!
What’s your favourite christmas film?
Jingle All the Way…err, I mean, Die Hard!
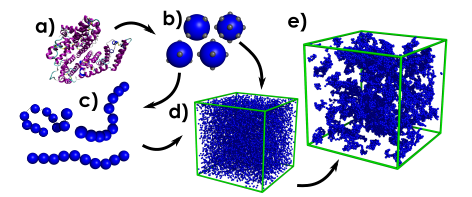
The work mentioned in this blog was performed with the support of Professor Lorna Dougan and financial support from the EPSRC, Grant code EP/P02288X/1

